The COVID-19 global pandemic has forced companies in the industry to develop medical devices to help manage the pandemic, but do you know what techniques are currently available and what are the differences between SARS-CoV-2 coronavirus testing? We explain it to you!
Once a person comes into contact with a virus, the virus multiplies using human cells as machinery and the immune system reacts to try to fight it by generating, among other things, specific antibodies against that virus.
In this regard, there are currently two types of SARS-CoV-2 coronavirus detection tests on the market:
1. VIRAL TESTS.
- Detection of ribonucleic acid (RNA):The genetic material of viruses can be DNA or RNA. In this case, the genetic material of SARS-CoV-2 is RNA.
In order to detect this genetic material, the PCR (Polymerase Chain Reaction) test is used.
- Detection of antigens:Antigens are foreign substances (external or internal) to the body that induce an immune response in our body and trigger the formation of antibodies.
To detect these antigens, which are nothing more than the proteins of the virus, what are known as “rapid antigen tests” are used.
2. ANTIBODY TESTS.
- Antibody detection: Antibodies, also known as immunoglobulins, are glycoproteins that are generated by our body to fight foreign substances. They are synthesized by a type of leukocyte called B lymphocyte and some of their functions are:
- Neutralize the action of the pathogen.
- Mark those places where the immune system has to act more strongly.
- To detect these immunoglobulins, so-called “serological tests” are used.
PCR
For the detection of the SARS-CoV-2 virus, PCR (Polymerase Chain Reaction) is a laboratory technique that consists, in a simplified way, in detecting a specific fragment of the genetic material of the virus.
The sample needed to perform this type of SARS-CoV-2 coronavirus detection tests is nasopharyngeal or saliva and what PCR allows is to “amplify”, that is, to make many copies, of that specific and known sequence of the virus found in the sample taken.
If that particular segment of the virus is not amplified, the test result is negative. Conversely, if that segment of the virus has been amplified, it will mean that the result is positive and, therefore, the person is a carrier of the virus.
For all these reasons, PCR is a diagnostic test, which can give the result in about 4-6 hours.
RAPID ANTIGEN TESTS
The rapid antigen tests detect, as the name suggests, the antigens of the virus, in this case the virus’ own proteins. This type of test therefore detects those substances that the body detects as foreign.
To obtain the results of this test, in most cases, a nasopharyngeal or saliva sample. must be taken. This sample is mixed with a reagent that allows the proteins to be released and placed on the well of a cassette containing the specific antibodies that bind to these antigens.
The antigen test is a prueba diagnóstica, so if the person is a carrier of the virus, the virus antigen will bind to the antibodies and the result will be positive. In the same way as with PCR, if the virus is not carried, there will be no antigen-antibody binding and, therefore, the result will be negative.
The advantage of this type of test is that they show the result in 10-15 minutes. However, they are not as sensitive as a PCR as they require a sample with a higher viral load because, unlike PCR, no “amplification” is performed.
SEROLOGICAL TESTS
Serological tests are those that detect the antibodies that the organism has generated before an infection. In the case of SARS-CoV-2, IgG and IgM antibodies, among others, are detected.
These antibodies appear in different periods of the infection, therefore, these tests allow us to detect in which phase of the infection we are (early, advanced or if we have passed the disease).
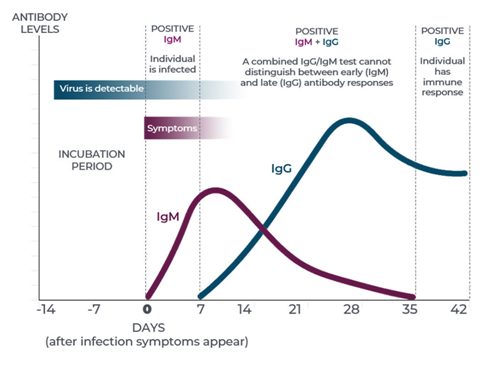 Source: Beckman Coulter Diagnostics
Source: Beckman Coulter Diagnostics
However, serological tests are not recommended as diagnostic tests for active COVID-19 infection since we may be infected and not have generated antibodies yet, but they are very useful as a tool for epidemiological studies and to study the effectiveness of vaccines.
There are different types of serological tests, but the most widely used at present for COVID-19 are ELISA and rapid tests. The fundamental differences between the two methods are as follows:
| METHOD | TIME | SAMPLE | DETECTION | ESPECIALIZATION | SENSIBILITY | AVERAGE PRICE |
| LATERAL FLOW (RAPID TEST) | 10 minutes | Drop of Blood | Cualitative | Not required | High | < 50 € |
| ELISA/ECLIA | 2 hours | Blood Sample | Semicuantitative | Required: devices and personal | Very High | 60-160 € |
In summary, there are many differences between SARS-CoV-2 coronavirus detection tests depending on what is to be detected, the time required to obtain the results, the use for which it is needed, etc.
| Detection | Sample | Time | Use | |
| TEST SEROLÓGICO | Anticuerpos | Sanguínea | -ELISA: Medio-Test rápido: Rápido | -Estudios seroprevalencia-Eficacia vacunas |
| SEROLOGICAL TEST/strong> | Antibodies | Blood | -ELISA: Medium -Rapid test: Fast |
-Epidemiological studies -Effectiveness of vaccines |
| ANTIGEN TEST | Virus antigens | Nasopharyngeal or saliva | Fast | Diagnostic |
| PCR | Virus RNA | Nasopharyngeal or saliva | Slow | Diagnostic |
Therefore, depending on the purpose of these SARS-CoV-2 coronavirus detection tests, it will be better to choose between one type or another.
Did you like this post? You can subscribe to our newsletter to receive blog updates and the latest news!