BIOLAN HEALTH has been marketing its antigen tests for the detection of COVID for a year now, and since this week it has been able to market its self-testing antigen tests in pharmacies. Until now, BIOLAN HEALTH has only had the opportunity to market the tests for professional use to private companies or social-health institutions, mainly exporting to countries outside Europe.
After a lot of hard work, it has finally obtained full quality assurance certification for its use as a self-test, issued by Notified Body 0318, following the rigorous audit carried out last April. This achievement allows the entity to sell in the pharmacy channel and thus offer the public a Km 0 product, developed and manufactured entirely in its own facilities.
Currently, the BIOLAN HEALTH self-test is on the list of 56 brands authorised by the health authorities for sale in Spanish pharmacies, the vast majority of which, around 50, are of Chinese origin.
It is a proprietary product, from development to manufacturing, the result of the significant investment effort that the BIOLAN group, to which BIOLAN HEALTH belongs, has made in the last two years and which has provided it with the production capacity for industrial scaling.

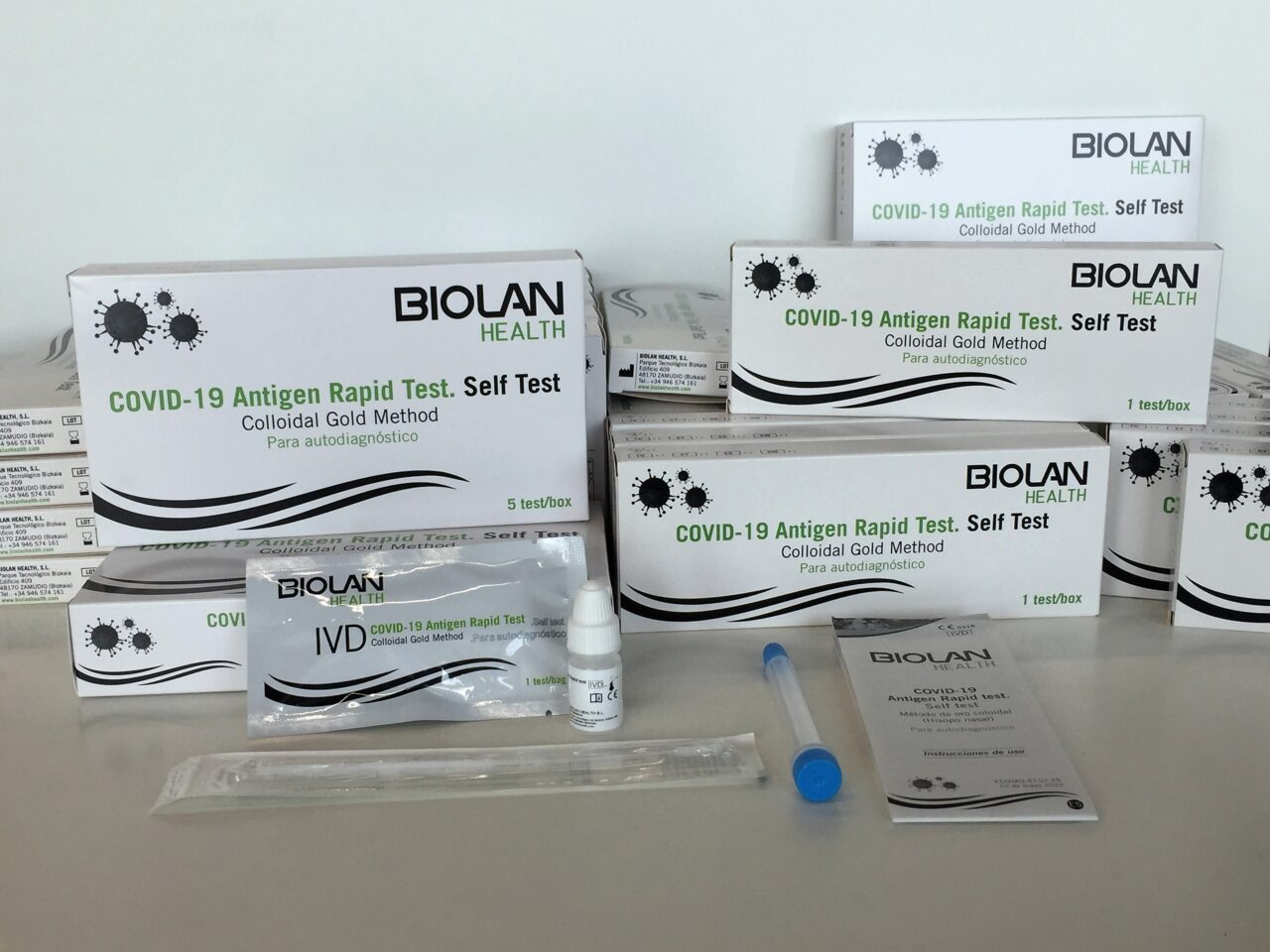
The new BIOLAN HEALTH antigen test to detect the SARS-CoV-2 virus, called COVID-19 Antigen Rapid Test-Self Test, is an immunochromatographic test for the qualitative detection of viral antigens. It is a rapid, easy-to-use test that detects part of the structure of the virus from a nasal sample and allows the identification of people who are infected by the virus that causes COVID-19 and who may be contagious. The fact that only a nasal swab is required, obtained in a less invasive and less uncomfortable way for the patient than nasopharyngeal swabbing, makes the test a clear advantage for the user. This rapid self-diagnostic antigen test guarantees a reliable and accurate result in 15-20 minutes, with validated values of 97.2% sensitivity and 98.1% specificity, which meet the criteria defined by the WHO, and therefore makes it a very useful tool for the early identification and isolation of potentially infectious COVID-19 positive cases, thus controlling the spread of the virus through easy interpretation of the results.
BIOLAN HEALTH offers pharmacies the self-diagnosis tests in 3 kit formats, of 1, 5 and 25 units. These kits include all the necessary material to carry out the tests in a simple and quick way. Pharmacies can purchase these tests by emailing pedidos@biolanhealth.com, which are being delivered within 48 hours.
BIOLAN HEALTH’s product line to fight the pandemic currently ranges from the detection of infection to the assessment of immunity to this infection. In addition to antigen tests for both professional use and self-testing through sales in pharmacies, BIOLAN HEALTH markets the serological rapid test for the detection of antibodies against COVID-19, which has been on the market since the beginning of 2021. This test has become a very useful tool for assessing the immunity of citizens naturally or derived from vaccination because it can detect neutralising antibodies, directed against a specific region of the S protein, which are those generated by vaccines, allowing the effectiveness of vaccines to be assessed effectively and reliably, something that not all rapid tests provide because they are not designed for this purpose.
BIOLAN HEALTH thus reinforces its commitment to the fight against COVID-19 and continues to apply its knowledge and devote significant resources to provide society with tools that enable better management of the current situation arising from the pandemic. BIOLAN HEALTH also has a manufacturing and export licence from the Spanish Medicines and Health Products Agency (AEMPS), as well as ISO 13485 certification that guarantees all quality standards.
Finally, BIOLAN HEALTH is part of the OSASUNBERRI platform, a platform that aims to provide an “INTEGRAL SOLUTION OF DIAGNOSIS, THERAPIES AND CARE SYSTEM TO STRENGTHEN THE KM 0 HEALTH SECTOR”. In this sense, the R&D, design and manufacture of these antigen tests has been developed in collaboration with multiple partners of this collaborative initiative, which strengthens the health sector in our environment.